Choline
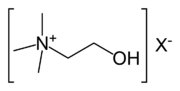
Choline is a water-soluble essential nutrient.[1][2][3][4][5] It is usually grouped within the B-complex vitamins. Choline generally refers to the various quaternary ammonium salts containing the N,N,N-trimethylethanolammonium cation.
These naturally-occurring ammonium salts are found in the lipids that make up cell membranes and in the neurotransmitter acetylcholine.
Contents |
History
Choline was discovered by Adolph Strecker in 1864 and chemically synthesized in 1866. In 1998 choline was classified as an essential nutrient by the Food and Nutrition Board of the Institute of Medicine (U.S.A.).
Chemistry
Choline is a quaternary saturated amine with the chemical formula (CH3)3N+CH2CH2OHX−, where X− is a counterion such as chloride (see choline chloride), hydroxide or tartrate. Choline chloride, in mixture with urea is used as a solvent (DES) and the salicylate salt is used topically for pain relief of aphthous ulcers.
Choline hydroxide
Choline hydroxide is one of the class of phase transfer catalysts which are used to carry the hydroxide ion into organic systems, and because of this it is considered a strong base. It is the least costly phase transfer catalyst, and is used as a cheap method of stripping photoresists in circuit boards. Choline hydroxide is not completely stable and it slowly breaks down into trimethylamine.
Role in humans
Physiology
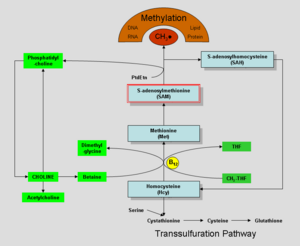
Choline and its metabolites are needed for three main physiological purposes: structural integrity and signaling roles for cell membranes, cholinergic neurotransmission (acetylcholine synthesis), and as a major source for methyl groups via its metabolite, trimethylglycine (betaine) that participates in the S-adenosylmethionine synthesis pathways.
Fish odor syndrome
Choline is a precursor to trimethylamine, which some persons are not able to break down due to a genetic disorder called trimethylaminuria. Persons suffering from this disorder may suffer from a strong fishy or otherwise unpleasant body odor, due to the body's release of odorous trimethylamine. A body odor will occur even on a normal diet – i.e., one that is not particularly high in choline. Persons with trimethylaminuria are advised to restrict the intake of foods high in choline; this may help to reduce the sufferer's body odor.Some studies have also shown that copper chlorophyll supplements have also been used to eliminate or greatly reduce the unpleasant odor.
Food sources of choline
The Adequate Intake of choline is 425 mg (milligrams) per day for adult women; higher for pregnant and breastfeeding women. The AI for adult men is 550 mg/day. There are also AIs for children and teens.[6]
| Choline and calories in some animal and plant foods | ||
|---|---|---|
| 5 ounces (142 g) raw beef liver | 473 mg choline | 192 calories[nb 1] |
| Large hardboiled egg | 113 mg choline | 78 calories[nb 2] |
| Half a pound (227 g) codfish | 190 mg choline | 238 calories[nb 3] |
| Half a pound of chicken | 149 mg choline | 270 calories[nb 4] |
| Quart of milk, 1% fat | 173 mg choline | 410 calories[nb 5] |
| A tablespoon (8 g) soy lecithin | About 250 mg choline | 60 calories[7] |
| A pound (454 grams) of cauliflower | 177 mg choline | 104 calories [nb 6] |
| A pound of spinach | 113 mg choline | 154 calories[nb 7] |
| A cup of wheat germ | 202 mg choline | 432 calories[nb 8] |
| Two cups (0.47 liters) firm tofu | 142 mg choline | 353 calories[nb 9] |
| Two cups of cooked kidney beans | 108 mg choline | 450 calories. [nb 10] |
| A cup of uncooked quinoa | 119 mg choline | 626 calories. [nb 11] |
| A cup of uncooked amaranth | 135 mg choline | 716 calories [nb 12] |
| A grapefruit | 19 mg choline | 103 calories[nb 13] |
| 3 cups (710 cc) cooked brown rice | 54 mg choline | 649 calories [nb 14] |
| A cup (146 g) of peanuts | 77 mg choline | 828 calories [nb 15] |
| A cup (143 g) of almonds | 74 mg choline | 822 calories [nb 16] |
Besides cauliflower, other cruciferous vegetables may also be good sources of choline.[8]
Choline and other nutrient values for many foods can be obtained online.[a 1]
Groups at risk for choline deficiency
Strict vegetarians who avoid all animal products, endurance athletes and people who drink a lot of alcohol may be at risk for choline deficiency and may benefit from choline supplements.[9]
In general, people who do not eat many whole eggs may have to pay close attention to get enough choline in their diets.[10] Studies on a number of different populations have found that the average intake of choline was below the Adequate Intake.[2][11]
The choline researcher Dr. Steven Zeisel wrote: "A recent analysis of data from NHANES 2003–2004 revealed that for [American] older children, men, women and pregnant women, mean choline intakes are far below the AI. Ten percent or fewer had usual choline intakes at or above the AI."[2]
Health effects of dietary choline
Choline deficiency may play a role in liver disease, atherosclerosis and possibly neurological disorders,[2] One symptom of choline deficiency is an elevated level of the liver enzyme ALT.[12]
It is particularly important for pregnant women to get enough choline, since low choline intake may raise the rate of neural tube defects in infants, and may affect their child's memory. One study found that higher dietary intake of choline shortly before and after conception was associated with a lower risk of neural tube defects.[13] If low choline intake causes an elevated homocysteine level, it raises the risk for preeclampsia, premature birth, and very low birth weight.[2]
Women who have diets richer in choline may have a lower risk for breast cancer,[14][15] but other studies found no association.[16][17]
There is some evidence that choline is anti-inflammatory. In the ATTICA study, higher dietary intake of choline was associated with lower levels of inflammatory markers.[18] A small study found that choline supplements reduced symptoms of allergic rhinitis.[19]
Despite its importance in the central nervous system as a precursor for acetylcholine and membrane phosphatidylcholine, the role of choline in mental illness has been little studied. In a large population-based study, blood levels of choline were inversely correlated with anxiety symptoms in subjects aged 46–49 and 70–74 years. However, there was no correlation between depression and choline level in this study.[20]
The Adequate Intake is intended to be high enough to be adequate for almost all healthy people.[21] Many people do not develop deficiency symptoms when consuming less than the Adequate Intake of choline.[2] The human body synthesizes some of the choline it needs, and people vary in their need for dietary choline.[22] In one study, premenopausal women were less sensitive to a low-choline diet than men or postmenopausal women.[22]
However, the Adequate Intake may not be enough for some people. In the same study, 6 out of 26 men developed choline deficiency symptoms while consuming the Adequate Intake (and no more) of choline.[22] The Adequate Intake was less than the optimal intake for the male subjects in another study.[23]
High dietary intake of choline was associated with an increased risk of colon adenomas (polyps), for women in the Nurses' Health Study. However, this could represent effects of other components in the foods from which choline was obtained.[24] Dietary choline intake was not associated with increased risk of colorectal cancer, for men in the Health Professionals Follow-up Study.[25]
Choline as a dietary supplement
The most often available choline supplement is lecithin, derived from soy or egg yolks, often used as a food additive. Phosphatidylcholine is also available as a supplement, in pill or powder form. Supplementary choline is also available as choline chloride, which comes as a liquid due to its hydrophilic properties. Choline chloride is sometimes preferred as a supplement because phosphatidylcholine can have gastrointestinal side effects.
It is well established that supplements of methyl group transfer vitamins B6, B12, folic acid reduce the blood titer of homocysteine and so may prevent heart disease.[26] Choline or betaine supplements also may reduce homocysteine.[27] Choline is a necessary source of methyl groups for methyl group transfer. Supplements of lecithin/choline were found to reduce heart disease in laboratory studies. The reduction in heart disease with lecithin supplements may however relate more to the cholesterol carrying capacity of lecithin than to the methyl group transfer role of choline.
Choline supplements are often taken as a form of 'smart drug' or nootropic, due to the role that the neurotransmitter acetylcholine plays in various cognition systems within the brain. Choline is a chemical precursor or "building block" needed to produce the neurotransmitter acetylcholine, and research suggests that memory, intelligence and mood are mediated at least in part by acetylcholine metabolism in the brain. In study on rats, a correlation was shown between choline intake during pregnancy and mental task performance of the offspring;[28] however, the same correlation has not been shown in humans.[29] However, this human study admits "Women in the current study consumed their usual diets. They were not eating choline-enriched diets and were not receiving choline supplementation. Therefore, our results indicate that choline concentrations in a physiologic range observed among women consuming a regular diet during pregnancy are not related to IQ in their offspring. We cannot rule out the possibility that choline supplementation could have an IQ effect."
The compound's quaternary amine renders it lipid insoluble which might suggest it would be unable to cross the blood-brain barrier. However, despite choline's lipid insolubility, a choline transporter exists that allows transport across the blood-brain barrier. The efficacy of these supplements in enhancing cognitive abilities is a topic of continuing debate.
The Food and Drug Administration (FDA) requires that infant formula not made from cow's milk be supplemented with choline.[30]
Due to its role in lipid metabolism, choline has also found its way into nutritional supplements which claim to reduce body fat; but there is little or no evidence to prove that it has any effect on reducing excess body fat or that taking high amounts of choline will increase the rate at which fat is metabolised.
Pharmaceutical uses
Choline is used in the treatment of liver disorders,[31][32] Alzheimer's disease,[33] and bipolar depression.[34]
Some studies show that as a supplement, choline is also used in treating hepatitis, glaucoma,[35] atherosclerosis, and, possibly, neurological disorders.[2]
Choline has also been proved to have a positive effect on those suffering from alcoholism.[36][37]
The current NIH funded research study COBRIT is gathering data regarding potential benefit of longterm citicoline treatment for recovery after traumatic brain injury.
Additional images
 Choline (C5H14NO+) |
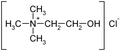 Choline chloride |
Choline hydroxide |
Notes
- ↑ The USDA Nutrients Database has choline content for many foods. If the USDA Nutrients Database doesn't list choline content for a food, try looking for a similar food in the database Choline Content of Common Foods. Then, look up that food in the USDA Nutrients Database.
See also
- Cytidine diphosphate choline
- Dimethylethanolamine
References
- ↑ Entry for "Beef, variety meats and by-products, liver, raw" in the USDA Nutrients database
- ↑ Entry for one large "Egg, whole, cooked, hard-boiled" in the USDA Nutrients database
- ↑ Entry for "Fish, cod, Atlantic, cooked, dry heat" in the USDA Nutrients database
- ↑ Entry for "Milk, lowfat, fluid, 1% milkfat, with added vitamin A and vitamin D" in the USDA Nutrients database
- ↑ Entry for "Milk, lowfat, fluid, 1% milkfat, with added vitamin A and vitamin D" in the USDA Nutrients database
- ↑ Entry for "Cauliflower, cooked, boiled, drained, with salt" in the USDA Nutrients database
- ↑ Entry for "Spinach, frozen, chopped or leaf, cooked, boiled, drained, without salt" in the USDA Nutrients database
- ↑ Entry for "Cereals ready-to-eat, wheat germ, toasted, plain" in the USDA Nutrients database
- ↑ Entry for "Tofu, firm, prepared with calcium sulfate and magnesium chloride (nigari) (1)" in the USDA Nutrients database
- ↑ Entry for "Beans, kidney, all types, mature seeds, cooked, boiled, without salt" in the USDA Nutrients database
- ↑ Entry for "Quinoa, uncooked" in the USDA Nutrients database
- ↑ Entry for "Amaranth, uncooked" in the USDA Nutrients database
- ↑ Entry for "Grapefruit, raw, pink and red, all areas" in the USDA Nutrients database
- ↑ Entry for "Rice, brown, long-grain, cooked" in the USDA Nutrients database
- ↑ Entry for "Peanuts, all types, raw" in the USDA Nutrients database
- ↑ Entry for 1 cup whole "Nuts, almonds" in the USDA Nutrients database
- ↑ Jane Higdon, "Choline", Micronutrient Information Center, Linus Pauling Institute
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 Zeisel SH, da Costa KA (November 2009). "Choline: an essential nutrient for public health". Nutrition Reviews 67 (11): 615–23. doi:10.1111/j.1753-4887.2009.00246.x. PMID 19906248.
- ↑ "Choline, PDRHealth
- ↑ "Choline" (An interview with Steven Zeisel, Editor-in-Chief of the Journal of Nutritional Biochemistry), Radio National Health Report with Norman Swan, Monday 17 April 2000
- ↑ "[1]" Dietary Reference Intakes for Thiamin, Riboflavin, Niacin, Vitamin B6, Folate, Vitamin B12, Pantothenic Acid, Biotin, and Choline (1998), Institute of Medicine.
- ↑ "Dietary Reference Intakes". Institute of Medicine. http://www.iom.edu/Activities/Nutrition/SummaryDRIs/~/media/Files/Activity%20Files/Nutrition/DRIs/DRI_Vitamins.ashx.
- ↑ "Bob's Red Mill soy lecithin - click on image of ingredients label". http://www.bobsredmill.com/soy-lecithin-granules.html.
- ↑ Gossell-Williams M, Fletcher H, McFarlane-Anderson N, Jacob A, Patel J, Zeisel S (December 2005). "Dietary intake of choline and plasma choline concentrations in pregnant women in Jamaica". The West Indian Medical Journal 54 (6): 355–9. PMID 16642650.
- ↑ "Choline". University of Pittsburgh Medical Center. http://nutritionservices.upmc.com/NutritionArticles/Vitamins/Choline.htm.
- ↑ Hasler CM (October 2000). "The changing face of functional foods". Journal of the American College of Nutrition 19 (5 Suppl): 499S–506S. PMID 11022999. http://www.jacn.org/cgi/pmidlookup?view=long&pmid=11022999.
- ↑ Bidulescu A, Chambless LE, Siega-Riz AM, Zeisel SH, Heiss G (2009). "Repeatability and measurement error in the assessment of choline and betaine dietary intake: the Atherosclerosis Risk in Communities (ARIC) study". Nutrition Journal 8: 14. doi:10.1186/1475-2891-8-14. PMID 19232103.
- ↑ "Micronutrient Information Center: Choline". Linus Pauling Institute. http://lpi.oregonstate.edu/infocenter/othernuts/choline/.
- ↑ Shaw GM, Carmichael SL, Yang W, Selvin S, Schaffer DM (July 2004). "Periconceptional dietary intake of choline and betaine and neural tube defects in offspring". American Journal of Epidemiology 160 (2): 102–9. doi:10.1093/aje/kwh187. PMID 15234930.
- ↑ Xu X, Gammon MD, Zeisel SH, et al. (November 2009). "High intakes of choline and betaine reduce breast cancer mortality in a population-based study". The FASEB Journal 23 (11): 4022–8. doi:10.1096/fj.09-136507. PMID 19635752.
- ↑ Xu X, Gammon MD, Zeisel SH, et al. (June 2008). "Choline metabolism and risk of breast cancer in a population-based study". The FASEB Journal 22 (6): 2045–52. doi:10.1096/fj.07-101279. PMID 18230680.
- ↑ Cho E, Holmes M, Hankinson SE, Willett WC (December 2007). "Nutrients involved in one-carbon metabolism and risk of breast cancer among premenopausal women". Cancer Epidemiology, Biomarkers & Prevention 16 (12): 2787–90. doi:10.1158/1055-9965.EPI-07-0683. PMID 18086790.
- ↑ Cho E, Holmes MD, Hankinson SE, Willett WC (February 2010). "Choline and betaine intake and risk of breast cancer among post-menopausal women". British Journal of Cancer 102 (3): 489–94. doi:10.1038/sj.bjc.6605510. PMID 20051955.
- ↑ Detopoulou P, Panagiotakos DB, Antonopoulou S, Pitsavos C, Stefanadis C (February 2008). "Dietary choline and betaine intakes in relation to concentrations of inflammatory markers in healthy adults: the ATTICA study". The American Journal of Clinical Nutrition 87 (2): 424–30. PMID 18258634. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=18258634.
- ↑ Das S, Gupta K, Gupta A, Gaur SN (March 2005). "Comparison of the efficacy of inhaled budesonide and oral choline in patients with allergic rhinitis". Saudi Medical Journal 26 (3): 421–4. PMID 15806211.
- ↑ Bjelland I, Tell GS, Vollset SE, Konstantinova S, Ueland PM (October 2009). "Choline in anxiety and depression: the Hordaland Health Study". The American Journal of Clinical Nutrition 90 (4): 1056–60. doi:10.3945/ajcn.2009.27493. PMID 19656836.
- ↑ "Using the Adequate Intake for Nutrient Assessment of Groups". Institute of Medicine. http://www.nap.edu/openbook.php?record_id=9956&page=106.
- ↑ 22.0 22.1 22.2 Fischer LM, daCosta KA, Kwock L, et al. (May 2007). "Sex and menopausal status influence human dietary requirements for the nutrient choline". The American Journal of Clinical Nutrition 85 (5): 1275–85. PMID 17490963. PMC 2435503. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=17490963.
- ↑ Veenema K, Solis C, Li R, et al. (September 2008). "Adequate Intake levels of choline are sufficient for preventing elevations in serum markers of liver dysfunction in Mexican American men but are not optimal for minimizing plasma total homocysteine increases after a methionine load". The American Journal of Clinical Nutrition 88 (3): 685–92. PMID 18779284. PMC 2637180. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=18779284.
- ↑ Cho E, Willett WC, Colditz GA, et al. (August 2007). "Dietary choline and betaine and the risk of distal colorectal adenoma in women". Journal of the National Cancer Institute 99 (16): 1224–31. doi:10.1093/jnci/djm082. PMID 17686825.
- ↑ Lee JE, Giovannucci E, Fuchs CS, Willett WC, Zeisel SH, Cho E (March 2010). "Choline and betaine intake and the risk of colorectal cancer in men". Cancer Epidemiology, Biomarkers & Prevention 19 (3): 884–7. doi:10.1158/1055-9965.EPI-09-1295. PMID 20160273.
- ↑ Verhoef P, Stampfer MJ, Buring JE, et al. (May 1996). "Homocysteine metabolism and risk of myocardial infarction: relation with vitamins B6, B12, and folate". American Journal of Epidemiology 143 (9): 845–59. PMID 8610698. http://aje.oxfordjournals.org/cgi/pmidlookup?view=long&pmid=8610698.
- ↑ Ueland PM (May 2010). "Choline and betaine in health and disease". Journal of Inherited Metabolic Disease. doi:10.1007/s10545-010-9088-4. PMID 20446114.
- ↑ Sternberg, Robert J. (2000). Handbook of intelligence. Cambridge, UK: Cambridge University Press. p. 77. ISBN 978-0-521-59648-0.
- ↑ Signore C, Ueland PM, Troendle J, Mills JL (April 2008). "Choline concentrations in human maternal and cord blood and intelligence at 5 y of age". The American Journal of Clinical Nutrition 87 (4): 896–902. PMID 18400712. PMC 2423009. http://www.ajcn.org/cgi/pmidlookup?view=long&pmid=18400712.
- ↑ "Requirements for Infant Formulas". FDA. http://www.fda.gov/RegulatoryInformation/Legislation/FederalFoodDrugandCosmeticActFDCAct/FDCActChapterIVFood/ucm107864.htm.
- ↑ Tolvanen T, Yli-Kerttula T, Ujula T, et al. (May 2010). "Biodistribution and radiation dosimetry of [(11)C]choline: a comparison between rat and human data". European Journal of Nuclear Medicine and Molecular Imaging 37 (5): 874–83. doi:10.1007/s00259-009-1346-z. PMID 20069295.
- ↑ Behari J, Yeh TH, Krauland L, et al. (February 2010). "Liver-specific beta-catenin knockout mice exhibit defective bile acid and cholesterol homeostasis and increased susceptibility to diet-induced steatohepatitis". The American Journal of Pathology 176 (2): 744–53. doi:10.2353/ajpath.2010.090667. PMID 20019186.
- ↑ Van Beek AH, Claassen JA (January 2010). "The cerebrovascular role of the cholinergic neural system in Alzheimer's disease". Behavioural Brain Research. doi:10.1016/j.bbr.2009.12.047. PMID 20060023.
- ↑ Ongür D, Prescot AP, Jensen JE, et al. (January 2010). "T2 relaxation time abnormalities in bipolar disorder and schizophrenia". Magnetic Resonance in Medicine 63 (1): 1–8. doi:10.1002/mrm.22148. PMID 19918902.
- ↑ Chan KC, So KF, Wu EX (January 2009). "Proton magnetic resonance spectroscopy revealed choline reduction in the visual cortex in an experimental model of chronic glaucoma". Experimental Eye Research 88 (1): 65–70. doi:10.1016/j.exer.2008.10.002. PMID 18992243.
- ↑ Klatskin G, Krehl WA (December 1954). "The effect of alcohol on the choline requirement. II. Incidence of renal necrosis in weanling rats following short term ingestion of alcohol". The Journal of Experimental Medicine 100 (6): 615–27. doi:10.1084/jem.100.6.615. PMID 13211918.
- ↑ Nery FG, Stanley JA, Chen HH, et al. (April 2010). "Bipolar disorder comorbid with alcoholism: a 1H magnetic resonance spectroscopy study". Journal of Psychiatric Research 44 (5): 278–85. doi:10.1016/j.jpsychires.2009.09.006. PMID 19818454.
External links
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||
|
||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||